STAB
(Sodium Triacetoxyborohydride)
Other Names:
Sodium triacetoxyhydroborate
General Information:
Structure:
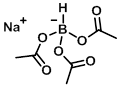
CAS Number: 56553-60-7
Molecular Weight: 211.96 g/mol
Appearance: White powder
Sodium triacetoxyborohydride (STAB) is a mild reducing agent that is commonly used in reductive aminations. STAB has the advantage over sodium cyanoborohydride (NaBH3CN) of not producing toxic side-pdts. One disadvantage of STAB is that it is H2O sensitive, not compatible with MeOH, and reacts slowly with EtOH and isopropanol. For these reasons STAB is typically used with aprotic solvents such as DCE, DCM, THF, dioxane, or toluene.
Common Uses:
Reagent for reductive aminations
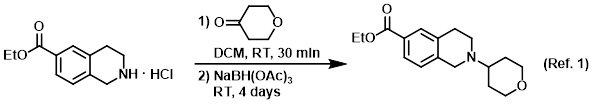
Procedure excerpt:
. . . The mixture was stirred at RT for 30 min, after which time was added NaBH(OAc)3 (12 g, 56 mmol). The reaction mixture was stirred at RT . . .
Reagent for the reduction of enamines
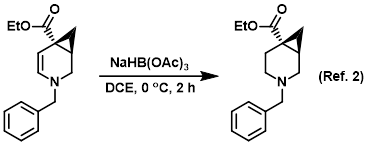
Procedure excerpt:
To a solution of the SM (crude) in DCE (50 mL) at 0 C was added NaBH(OAc)3 (3.6 g, 17.1 mmol). The reaction mixture was stirred at 0 C for 2 h, after which time . . .
Safety:
Care should be taken when forming sodium triacetoxyborohydride (STAB) in situ from NaBH4 and AcOH because H2 gas forms. Work should be done under an inert atmosphere.
References:
1) Patent Reference: WO2016014463, page 119, ![]() (6.7 MB)
(6.7 MB)
2) Patent Reference: WO2016014463, page 64, ![]() (6.7 MB)
(6.7 MB)
3) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis; Oxidizing and Reducing Reagents
4) Wikipedia: Sodium triacetoxyborohydride (link)
5) Wikipedia: Sodium cyanoborohydride (link)
6) www.sigmaaldrich.com: Sodium triacetoxyborohydride (link)
Related Books:
